Hydrogen fluoride
| Hydrogen fluoride | |
|---|---|
 |
|
 |
|
| Identifiers | |
| CAS number | 7664-39-3 |
| PubChem | 16211014 |
| RTECS number | MW7875000 |
| Properties | |
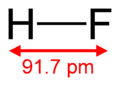
| Molecular formula | HF |
| Molar mass | 20.00634 g/mol |
| Appearance | colorless gas |
| Density | 1.15 g/l, gas (25 °C) 0.99 g/mL, liquid (19.5oC) |
| Melting point |
−83.6 °C, 190 K, -118 °F |
| Boiling point |
19.5 °C, 293 K, 67 °F |
| Solubility in water | miscible |
| Acidity (pKa) | 3.2 |
| Refractive index (nD) | 1.00001 |
| Structure | |
| Molecular shape | Linear |
| Dipole moment | 1.86 D |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−13.66 kJ/g (gas) −14.99 kJ/g (liquid) |
| Standard molar entropy S |
8.687 J/g K (gas) |
| Hazards | |
| NFPA 704 |
 0
4
1
|
| Related compounds | |
| Other anions | Hydrogen chloride Hydrogen bromide Hydrogen iodide |
| Other cations | Sodium fluoride |
| Related compounds | Hydrofluoric acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Hydrogen fluoride is a chemical compound with the formula HF. It is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers (e.g. Teflon). HF is widely used in the petrochemical industry and a component of many superacids. HF boils just below room temperature whereas the other hydrogen halides condense at much lower temperatures. Unlike the other hydrogen halides, HF is lighter than air and it is particularly penetrating, which can damage the lungs. Aqueous solutions of HF, called hydrofluoric acid, are strongly corrosive.
Contents |
Structure
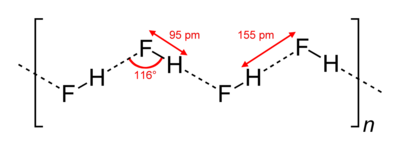
HF forms orthorhombic crystals, consisting of zig-zag chains of HF molecules. The HF molecules, with a short H–F bond of 0.95 Å, are linked to neighboring molecules by intermolecular H–F distances of 1.55 Å.[1]
Liquid HF also consists of chains of HF molecules, but the chains are shorter, consisting on average of only five or six molecules.[2] The higher boiling point of HF relative to analogous species, such as HCl, is attributed to hydrogen bonding between HF molecules, as indicated by the existence of chains even in the liquid state.
Acidity
The acidity of hydrofluoric acid solutions vary with concentration owing to hydrogen-bond interactions of the fluoride ion. Dilute solutions are weakly acidic with an acid ionization constant Ka = 6.6 x 10-4 (or pKa = 3.18),[3] in contrast to corresponding solutions of the other hydrogen halides which are strong acids. Concentrated solutions of hydrogen fluoride are much more strongly acid than implied by this value, as shown by measurements of the Hammett acidity function H0[4] (or “effective pH”). For 100%, HF has an H0, estimated to be between -10.2 and -11, which is comparable to the value -12 for sulfuric acid.[5][6]
In thermodynamic terms, HF solutions are highly non-ideal, with the activity of HF increasing much more rapidly than its concentration. The weak acidity in dilute solution is sometimes attributed to the high H—F bond strength, which combines with the high dissolution enthalpy of HF to outweigh the more negative enthalpy of hydration of the fluoride ion [7] However Giguère and Turrell[8][9] have shown by infrared spectroscopy that the predominant solute species is the hydrogen-bonded ion-pair [H3O+•F−], which suggests that the ionization can be described as a double equilibrium: H2O + HF = [H3O+•F−] = H3O+ + F−, with the first equilibrium to the right and the second to the left. This means that HF is extensively dissociated, but that the tight ion pairs reduce the thermodynamic activity coefficient of H3O+, so that the solution is effectively less acid.[10]
In concentrated solution, the additional HF causes the ion pair to dissociate with formation of the hydrogen-bonded hydrogen difluoride ion.[8],[10] [H3O+•F-] + HF = H3O+ + HF2− The increase in free H3O+ due to this reaction accounts for the rapid increase in acidity, while fluoride ions are stabilized (and become less basic) by strong hydrogen bonding to HF to form HF2-. At the limit of 100% liquid HF, there is autoionization 2 HF = H2F+ + F- to form an extremely acidic solution (H0 = -11).
The acidity of anhydrous HF can be increased even further by the addition of Lewis acids such as SbF5, which can reduce H0 to -21.[5],[6]
Production and uses
Hydrogen fluoride is produced as a side product of the extraction of the fertilizer precursor phosphoric acid from various minerals. This theme is developed in the article on hydrofluoric acid.
The anhydrous compound hydrogen fluoride is more commonly used than its aqueous solution, hydrofluoric acid. HF serves as a catalyst in alkylation processes in oil refineries. A component of high-octane gasoline called "alkylate" is generated in Alkylation units that combine C3 and C4 olefins and isobutane to generate gasoline.[11]
HF is a reactive solvent in the electrochemical fluorination of organic compounds. In this approach, HF is oxidized in the presence of a hydrocarbon and the fluorine replaces C–H bonds with C–F bonds. Perfluorinated carboxylic acids and sulfonic acids are produced in this way.[11]
Hydrogen fluoride is an important catalyst used in the majority of the installed linear alkyl benzene production in the world. The process involves dehydrogenation of n-paraffins to olefins, and subsequent reaction with benzene using HF as catalyst.
Elemental fluorine, F2, is prepared by electrolysis of a solution of HF and potassium bifluoride. The potassium bifluoride is needed because anhydrous hydrogen fluoride does not conduct electricity. Several million kilograms of F2 are produced annually.[12]
Health effects
Upon contact with moisture, including tissue, hydrogen fluoride immediately converts to hydrofluoric acid, which is highly corrosive and toxic, and requires immediate medical attention.
References
- ↑ Johnson, M. W.; Sándor, E.; Arzi, E. (1975). "The Crystal Structure of Deuterium Fluoride". Acta Crystallographica B31: pages 1998–2003. doi:10.1107/S0567740875006711.
- ↑ Mclain, Sylvia E.; Benmore, CJ; Siewenie, JE; Urquidi, J; Turner, JF (2004). "On the Structure of Liquid Hydrogen Fluoride". Angewandte Chemie, International Edition 43 (15): 1952–55. doi:10.1002/anie.200353289. PMID 15065271.
- ↑ R.H. Petrucci, W.S. Harwood and F.G. Herring “General Chemistry” (8th edition, Prentice Hall 2002) p.678
- ↑ H.H. Hyman et al., J. Amer. Chem. Soc. 79, 3668 (1957) "The Hammett acidity function H0 for HF aqueous solutions".
- ↑ 5.0 5.1 W.L. Jolly “Modern Inorganic Chemistry” (McGraw-Hill 1984), p.203
- ↑ 6.0 6.1 F.A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry (John Wiley and Sons: New York, 1988. ISBN 0471849979) p.109
- ↑ C.E. Housecroft and A.G. Sharpe “Inorganic Chemistry” (Pearson Prentice Hall, 2nd edn 2005), p.170.
- ↑ 8.0 8.1 Giguère, Paul A. and Turrell, Sylvia (1980). "The nature of hydrofluoric acid. A spectroscopic study of the proton-transfer complex H3O+...F−". J. Am. Chem. Soc. 102: 5473. doi:10.1021/ja00537a008.
- ↑ Radu Iftimie, Vibin Thomas, Sylvain Plessis, Patrick Marchand, and Patrick Ayotte (2008). "Spectral Signatures and Molecular Origin of Acid Dissociation Intermediates". J. Am. Chem. Soc. 130 (18): 5901. doi:10.1021/ja077846o. PMID 18386892.
- ↑ 10.0 10.1 F.A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry p.104
- ↑ 11.0 11.1 J. Aigueperse, P. Mollard, D. Devilliers, M. Chemla, R. Faron, R. Romano, J. P. Cuer, “Fluorine Compounds, Inorganic” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005
- ↑ M. Jaccaud, R. Faron, D. Devilliers, R. Romano “Fluorine” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- "ATSDR - MMG: Hydrogen Fluoride". Retrieved May 14, 2006
- Barbalace, Kenneth. "Chemical Database - Hydrogen Fluoride. EnvironmentalChemistry.com". 1995 - 2006. Retrieved May 14, 2006
- Honeywell, Industrial Fluorines G525-521, "Recommended Medical Treatment for Hydrofluoric Acid Exposure"
|
|||||